

Cycads are dioecious plants, having separate male and female individuals.
Male plants form cones that produce pollen, and female plants
form cones that produce seeds. Typically, cycads are insect-pollinated
in habitat. Studies have shown that there is a very close relationship
between specific insects and the species they pollinate. If these
insects are not present in a cultivated location, seeds will rarely
be produced. In this case, the female plant needs to be hand-pollinated
to produce viable seeds. In order to hand-pollinate your cycads,
you must collect the pollen from male plants and deposit it into
a receptive female cone. In another article, I explained how to
collect and store pollen. In this article, I will talk about the
different ways to hand-pollinate cycads so the number of viable
seeds produced is maximized.
Each cycad species has a very specific time of year that it produces
male and female cones. The timing of cone production will vary
from location to location throughout the world, and even at the
same location, the timing may vary by a few weeks each year, depending
on local weather conditions. Most species will have male cones
shedding pollen and female cones becoming receptive over the same
three-week period each year. The only genus that I have found
that doesn't conform to this rule is Ceratozamia. Ceratozamia
species drop pollen and become receptive over a longer period
of time throughout the spring.
When female cones are receptive to pollination, some of the cone
scales will loosen and form openings to let insects in. In habitat,
these insects will already have pollen clinging to their legs.
Even though this is not a firm rule, female cones will normally
be receptive for five to seven days. After the period of receptivity
is over, the cone scales will close up, and once this happens,
the cone cannot be pollinated. If the cone is pollinated correctly,
the seeds will grow and the cone will get larger. Encephalartos
seeds are different from those of other genera because they are
full-sized whether they have been pollinated or not. When it comes
time to germinate the seeds, this makes it difficult to tell if
you have produced good Encepha-lartos seeds, so you must cut a
few open to inspect for the presence of embryos.
Female cones contain ovules that if fertilized become seeds. Each
day while the cone is receptive, these ovules will secrete a sticky
substance called the "pollination droplet" from the
micropyle, and later the same day, will start to dry out, pulling
the liquid back into the ovule. If any pollen is stuck to the
droplet, it, too, is pulled into the ovule, and that ovule can
become a seed. Several scientists have written about pollen receptivity
and cycad-insect relationships, but I will try to give you a few
generalizations that will help you to be more successful at hand-pollination.
Male and female cones often produce a scent to attract certain
insects, and the cones can heat up as much as 15 degrees above
the ambient temperature, helping to volatilize the chemical scent.
To an insect that can see infrared light, a female cone looks
much like a light bulb at night, attracting many insects. Insects
are thought to be able to see receptive female cones as far away
as a quarter mile. Each day while a cone is receptive, the ovules
will start to secrete the aromatic chemical(s) late in the day
around 7pm. The cones will heat up during the night and will attract
insects. As the outside temperatures start getting hot in late
morning, the drop of liquid will start to dry up, and will be
sucked into the ovule. I have found that the very best time to
hand-pollinate cycads is first thing in the morning, while it
is still cool. I have had great success pollinating at this time,
and have known other people in my area, who pollinated in the
afternoon, that had very bad results pollinating the same species.
Hand-pollination is not difficult. As long as pollen gets into
the cone while it is receptive, good seeds can be produced. It
is really as simple as that. There are two basic methods for hand
pollination, the dry method and the wet method. The dry method
is as easy as putting the dry pollen in the cone. There are tools
that are made just for pollination, but what I do is cut half
the end off of a drinking straw so that it is shaped like a ladle,
and scoop the pollen with that. I make the end so thin that it
can be placed in-between the cone scales, and then drop the pollen
into the cone. Sometimes I blow it into the cone. Once I have
done that, I thump the cone lightly with my finger to get the
pollen to run down throughout the cone. Some cycad experts like
to mix their pollen with flour, or other inert substances to make
it go further. This can be important when working with small zamia
species, like Zamia purpurea, that produce very small quantities
of pollen. I just try to be careful and get all the pollen I can
into the cone without spilling any, and I have never had a problem
producing seeds on the small zamias. The wet method involves mixing
the pollen with water and injecting that mixture into the cone.
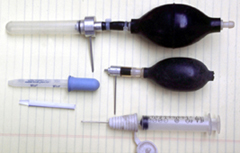
The commonly used tools are turkey basters, syringes, and eye
droppers, but anything along these lines can be used (Fig. 1).
The wet method is best when you only have a small amount of pollen
available, or if you are working with very large cones such as
those of Encephalartos and Lepidozamia. People don't realize how
much pollen it actually takes to produce good seeds. The smallest
amount of pollen the average person can see, would probably be
enough to easily produce 100 to 1000 seeds. If I have all the
pollen I need, I like to use enough to make the mixture fairly
cloudy, maybe half a teaspoon of pollen for every two ounces of
water. Sometimes this is not possible, but a little pollen can
go a long way.
There is a lot of debate on how many times a female cone should
be pollinated during its receptive period. There is some evidence
that ovules from one end of the cone to the other are receptive
at different times; if this is the case, pollen would have to
be present each day the cone appears to be receptive. Some people
feel that the cone should be pollinated each day. Some say that
two or three times is enough. I have found that it all depends
on the method being used and the time of day. For optimum results,
you want pollen to be pulled into all the ovules every day they
go through their cycle. Of course, this probably rarely happens.
When using the wet method, most of the pollen will run down through
the cone and exit out the bottom. There is obviously some pollen
that may stick to the inside of the cone, but this can get washed
out easily, especially in Florida, when it can sometimes rain
every afternoon. It is possible that if the wet method were used
at 2pm, and it rained at 4pm, pollination might be unsuccessful.
When using the wet method, I like to pollinate cones two or three
times just to make sure. I have found that using the dry method
only once during the first day of receptivity will do the job
very well, as long as enough pollen is used. There will be plenty
of pollen to stick to the drops of liquid on the ovules each day
it is receptive. Each day, go back to the plant, and thump the
cone again to get the pollen worked in. The pollen will be viable
for several days if it is fresh, so new pollen is not needed.
Multiple applications may be needed if it rains hard, if the pollen
is on the verge of becoming inviable, or if you are in a part
of the world where it gets 110F at the same time the cones are
receptive.
Most of the genera are pollinated in the same manner. Cracks will
form between some of the cone scales, and these patterns will
vary depending on the genus and species that is involved. Zamias
will typically crack at the top of the cone and sometimes near
the bottom. Occasionally there will be open cone scales throughout
the cone. I have found that the wet method and the dry method
both work equally as well with most zamias. Fig. 2a shows a Zamia
pumila cone that is receptive, being pollinated by the dry method
using the cut off drinking straw. Fig. 2b shows the wet method
of pollination with a syringe on the same cone.
 |
 |
 |
| Fig. 1 Various tools of the hand pollination trade, including bulbs, syringes, eyedroppers, and cut off straws. | Fig. 2a Pollinating Zamia pumila using the dry method, a cut off straw is employed | Fig. 2b shows the wet method of pollination with a syringe on the same cone. |
In both cases, I apply pollen into all of the cone scales at
the top to make sure it gets down in the cone as uniformly as
possible. If I have plenty of pollen, and there are more cone
scales that are open, I will try to get pollen in all of the other
cone scales as well, just to make sure. Most of the other genera
with small cones are pollinated in this manner. These would be
Chigua, Bowenia, and the smaller Macrozamia species.
Ceratozamia cones look much like Zamia cones, but all the cone
scales will open up to be receptive to pollen. I have found that
a week before the cone becomes receptive, there will be an obvious
color change between the cone scales. A red or orange color will
become obvious and then the cone scales will start to loosen.
You should be able to move the cone scales back and forth a little
to form areas that you can apply the pollen. Fig. 3 shows a receptive
cone of Ceratozamia hildae; note the orange color between the
cone scales. Even though both methods will work on ceratozamias,
I have had much better success using the dry method. I apply the
pollen to each cone scale from top to bottom, and then run my
finger over the top of the scales to work the pollen in. I do
this three or four times around the entire cone, so that I know
at least some pollen got into every crack. I would like to mention
that ceratozamias are usually receptive in the spring, but sometimes
they produce cones at an off time and appear to be receptive in
the fall. I have found that these cones do not typically produce
good seeds, even though everything looks right. After a few years
of unsuccessfully pollinating Ceratozamia cones during this off
time, I took three apparently receptive cones and cut them apart.
None of these cones had ovules that were secreting any liquid.
I now cut off any female cones if they are produced during this
off season to conserve the plant's energy.
A section consisting of the top 25% of Encephalartos cone scales
are sterile, the remaining 75% fertile. The sterile cone scales
will be about half the size of the fertile cone scales. Encephalartos
cones will crack open in the area between the sterile cone scales
and the fertile cone scales. These cones are so large that the
wet method is best because it is difficult to get the dry pollen
all the way down to the bottom of the cone. Species of Lepidozamia,
Microcycas, and the larger macrozamias are in this same category.
The dry method can work, but I have found that only the top seeds
become fertile unless you really work hard to get the pollen down
throughout the cone. Fig. 4 shows an Encephalartos ferox cone
being pollinated with the wet method. Some people prefer to use
the dry method to pollinate the blue Encephalartos species. These
species do not get much rain in habitat and can't handle a lot
of extra moisture. I know of people that have rotted the cones
of blue species because they used too much water to attempt the
pollination.
|
|
 |
 |
| Fig. 3 Color change between cone scales is typical of receptive Ceratozamia species like this C. hildae. | Fig. 4 Pollinating Encephalartos ferox with the wet method often helps disperse pollen throughout large cones. | Fig. 5 Stangeria cone scales are slightly loose and can be pulled down when the cone is receptive to pollen. |
Stangeria eriopus cones are different because from a distance
they don't ever appear to be receptive. Cone scales will start
to show a slight yellow color, and begin to loosen. In order to
find out if the cone is receptive, you must be able to pull down
on the cone scale a little (Fig. 5). If it does not pull down,
it is not ready. The cone scale should be easily pulled down to
make a nearly 90-degree-angle to the cone axis. The top of the
cone scale's underside will appear smooth and green. If you apply
dry pollen to that smooth area, the pollen will slide down into
the cone. Try doing this to every cone scale if possible. Either
pollination method will work with stangerias, but I prefer the
dry method.
Cycas species are completely different from all of the other genera
of cycads because the female cones are actually a loose cluster
of sporophylls instead of a tight cone. Cycas revoluta is the
most common Cycas in our landscapes. Fig. 6a shows a female Cycas
revoluta cone before it becomes receptive. Fig. 6b shows the same
cone at receptivity. These cones will open like a flower for about
five days and will then close up again. Some people will take
a male cone and shake it on top of the female cone, and almost
every ovule will be pollinated. I use my straw and just sprinkle
the pollen over the ovules sparingly. As all parts of cycads are
poisonous, so is the pollen. I don't like to get any pollen on
me, nor do I want to breathe in any. When using any large amount
of pollen, you may want to wear gloves, goggles, and a mask or
respirator so that you don't breathe in the pollen. Some Cycas
species will not open like Cycas revoluta. It is harder to tell
when cones of these species are receptive. Cycas taitungensis,
commonly grown in my area, does not open up for pollination. If
you get close to the female cone when it is receptive, you can
smell the liquid being produced. If you put your hand between
the sporophylls, the sticky substance being secreted can be felt.
When I see that this species is receptive, I will drop dry pollen
throughout the cluster, and work it in good by ruffling up the
sporaphylls.
 |
 |
| Fig 6a Female cone of Cycas revoluta before it becomes receptive to pollen | Fig 6b The same cone during receptivity to pollen. |
Dioon cones are probably the most difficult to pollinate. When a female Dioon cone is receptive, only a layer of cone scales at the bottom of the cone will drop down. On a large plant, this may go totally unnoticed because the cone is held close to the crown. Fig. 7a shows a receptive cone of Dioon mejiae with the lower cone scales down. The problem is: how do you get pollen up into the cone? Dioons are also naturally insect-pollinated, so it is easy for insects to crawl up into the cone with pollen on their legs. There are many methods to pollinate a Dioon cone. Some people take the plastic tubing used for aerating tropical fish tanks, and run that up into the cone. They will take dry pollen and blow it up into the tube, or squirt a liquid mixture using the tube and a baster or syringe. Dioon leaves are very prickly and in order to get the tubing into the crack, you may need to remove some leaves. I used to inject a liquid solution in three or four places in the sides of the cone with my syringe. This worked very well, but would sometimes miss some of the upper seeds if I started too far down the cone. The best method I have found is to remove the top of the cone to expose the ovules, apply pollen (either wet or dry) to the top portion and let it run down into the cone. Once the cone is pollinated, the top can be placed back on the cone, and it will usually seal itself back on. This is important because you don't want too much water getting into the cone from rain or irrigation that can rot the cone. The top of the Dioon cone is also sterile. The cone has a central stem that runs up and down throughout the cone. You want to take a knife and sever the central stem just at the point where the ovules start. Fig. 7b shows the perfect spot to cut the cone. If you cut it in the correct spot, the entire top will come off in one piece. This may take a little practice, but after you have done it a couple of times, it is much easier to see where you need to make the cut. In Fig. 7c, I am dropping dry pollen into the open top of the Dioon cone.
 |
 |
 |
| Fig 7a Pollinating a Dioon mejiae cone, lower cone scales open, indicating receptivity to pollen | Fig 7b The position where the top of cone should be removed for hand pollination | Fig 7c Dropping dry pollen into the cone with its top removed. |
I have shown everyone how to hand- pollinate cycad cones, but
with this knowledge comes great responsibility. It is important
to pollinate our cycads to help produce more plants for the world.
All cycads are on the endangered species list. For this reason,
it is important only to collect pollen from a certain species,
and use it on the same species. Even though it is possible to
make hybrids, a person serious about conservation will make sure
not to produce hybrids that might be misidentified as a true species
by someone else later on. Another caution I should mention is
that just because a cycad will produce a cone, or more than one
cone, it is not advisable to pollinate all these cones. Producing
a cone and nourishing the developing seeds takes a toll on the
female plant. As much as 60% of the starch content of the stem
can be used up for the production of seeds. Cycads typically don't
produce female cones for the first time until they are much older
and larger than male plants first forming pollen cones. On average,
a female plant will be two or three years older than a male plant
when it produces a its first cone. Many collectors will not pollinate
the first female cone produced and will just cut it off to save
the plant's energy. I have known several people who killed their
female plants by pollinating them the first time they coned. This
has also happened in the case of some species, especially multiple-
headed zamias, that produce multiple cones on the same plant.
In nature, perhaps only one of these cones will be successfully
pollinated, but in cultivation, with hand-pollination, it is easy
to produce seeds on every cone. Depending on the stem mass of
the plant and how many cones it produces, I usually remove half
or more of the cones and only pollinate one or two, just to make
sure the plant lives to produce more seeds another day.
I hope this article will help you pollinate your cycads. All cycads
are endangered, and whenever we can produce more cycads, it will
benefit the world. Some species are not able to produce viable
seeds in habitat because the insect pollinators are no longer
present or male plants are not within pollinating distance of
female plants. If only one person who reads this article will
now able to produce viable seeds on a plant that otherwise would
not have, writing this article will have been worthwhile.
